First Quarter Ends with 5.41% Average Price Increase
FAYETTEVILLE, NY, UNITED STATES, April 19, 2022 /EINPresswire.com/ — The first quarter of 2022 was active with drug manufacturers as hundreds of single-source brand name drugs, as determined by the Centers for Medicare and Medicaid Services, had price increases. During this period, 700 brands rose an average of 5.41%, with the majority of the brands, 640, taking price hikes in January. The overall annual inflation rate in the United States for 2021, using the Consumer Price Index (CPI-U) data provided by the US Department of Labor Bureau of Labor Statistic, was 7.0% and 1.4% for 2020, to provide context for the drug price increases.
Price increases ranged from a low of 0.75% for Adakveo [Novartis], used to treat sickle cell disease in patients 16+ years of age, to a high of 105.93% for Contrave [Currax], used to help manage weight in obese or overweight adults. In addition to Contrave, four other brands had price increases of 25 percent or more: Atgam [Pfizer], used to treat moderate to severe aplastic anemia at 25.00%, Metopirone [HRA Pharma], used in the diagnosis of certain problems of the adrenal glands at 25.00%, Tudorza Pressair [AstraZeneca], used to treat chronic obstructive pulmonary disease at 43.05%, and Loreev XR [Almatica Pharma], used to treat anxiety disorders at 49.12%.
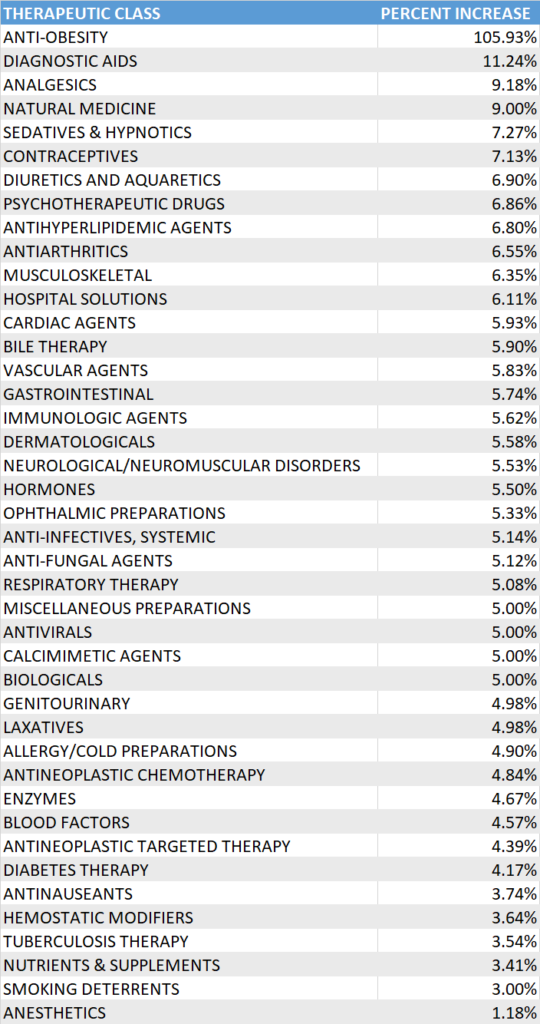
When grouping brands into their respective therapeutic class, anti-obesity had the highest percent increase at 105.93%, as noted above with Contrave being the only drug in this specific therapeutic class to have a price increase, followed by diagnostic aids and analgesics at 11.24% and 9.18% respectively. See chart for the complete list of therapeutic classes with price increases.
Please note that these price changes affect list prices, or Wholesale Acquisition Cost* (WAC), that are set by the drug manufacturers without taking into account rebates, insurance, and other discounts that may be available.
Source
AnalySource® as of April 6, 2022 – Reprinted with permission by First Databank, Inc. All rights reserved. © 2022
* First Databank, Inc Drug Pricing Policy: https://www.fdbhealth.com/drug-pricing-policy
About DMD America, Inc
AnalySource® is a registered trademark and drug pricing data solution service of DMD America, Inc. Since 1996, data has been made available in cooperation with First Databank, Inc. a subsidiary of the Hearst Corporation. Our service is licensed by subscription, with global clients including biotech, pharmaceuticals, government agencies, consultancies, academia, and more.
About First Databank (FDB)
First Databank (FDB) is the leading provider of drug and medical device knowledge that helps healthcare professionals make precise decisions. We empower our information system developer partners to deliver valuable, useful, and differentiated solutions used by millions of clinicians, business associates, and patients every day. For more than four decades, our medical knowledge has helped improve patient safety, operational efficiency, and healthcare outcomes. For a complete look at our solutions and services, please visit www.fdbhealth.com.
Eric Tedford
DMD America, Inc
+1 315-671-4208
email us here
